Table of Contents
How Effective is Acriptega in Managing HIV Infection?
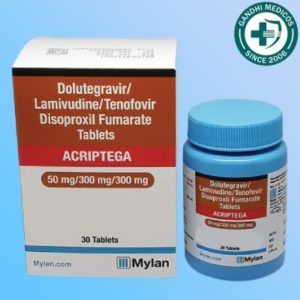 In the world of HIV treatment, the development of new medications and therapeutic approaches is essential for improving the quality of life for those affected by this condition. One such innovation is Acriptega, a tablet that combines Dolutegravir, Lamivudine, and Tenofovir disoproxil fumarate. This potent combination is manufactured by Roche Products India Pvt Ltd and is distributed under the brand name Mylan Pharmaceuticals Pvt Ltd. But how effective is Acriptega in managing HIV infection? In this comprehensive article, we will explore Acriptega’s composition, its common side effects, and its overall effectiveness in treating HIV.
In the world of HIV treatment, the development of new medications and therapeutic approaches is essential for improving the quality of life for those affected by this condition. One such innovation is Acriptega, a tablet that combines Dolutegravir, Lamivudine, and Tenofovir disoproxil fumarate. This potent combination is manufactured by Roche Products India Pvt Ltd and is distributed under the brand name Mylan Pharmaceuticals Pvt Ltd. But how effective is Acriptega in managing HIV infection? In this comprehensive article, we will explore Acriptega’s composition, its common side effects, and its overall effectiveness in treating HIV.
Composition of Acriptega Tablet
- Acriptega stands out due to its unique combination of active ingredients. Each tablet contains:
- Dolutegravir (50mg): Dolutegravir is an integrase inhibitor that prevents the integration of the HIV virus into human DNA, thus blocking its replication.
- Lamivudine (300mg): Lamivudine is a nucleoside reverse transcriptase inhibitor (NRTI) that hinders the reverse transcription of viral RNA into DNA, further suppressing HIV replication.
- Tenofovir disoproxil fumarate (300mg): Tenofovir is a nucleotide reverse transcriptase inhibitor (NtRTI) that complements Lamivudine’s action by inhibiting the reverse transcription process.
This potent combination targets various stages of the HIV life cycle, making it highly effective in combating the virus.
Dosing and Strength
Acriptega tablets come in a fixed-dose combination, with each tablet containing 50mg of Dolutegravir, 300mg of Lamivudine, and 300mg of Tenofovir disoproxil fumarate. This standardized dose ensures consistent and reliable treatment for individuals living with HIV.
Common Side Effects
Like any medication, Acriptega may have side effects, though not everyone experiences them. It’s essential to be aware of these side effects when taking this medicine.
Common side effects of this medicine include:
 Headache
Headache- Fever: Some
- Abdominal Pain
- Weakness
- Diarrhea and Nausea
- Vomiting
- Joint Pain:
- Insomnia (Difficulty in Sleeping)
- Gastrointestinal Disturbance
- Dizziness:
- Abnormal Dreams:
- Depression and Anxiety:
- Itching and Rash:
- Decreased Phosphate Level in Blood:
- General Discomfort
- Cough
- Increased Transaminase Level in Blood
- Hair Loss
- Muscle Disorders
It’s important to note that these side effects may vary in severity from person to person, and not everyone will experience them. Additionally, the benefits of Acriptega in managing HIV often outweigh the potential side effects.
Effectiveness of Acriptega in HIV Treatment
The effectiveness of Acriptega in managing HIV infection cannot be understated. The combination of Dolutegravir, Lamivudine, and Tenofovir disoproxil fumarate targets the virus at multiple stages of its life cycle, making it highly potent in suppressing viral replication. This not only helps reduce the viral load in the body but also contributes to maintaining a healthy immune system.
Furthermore, the fixed-dose combination of these three active ingredients simplifies treatment regimens, enhancing patient adherence. Consistency in taking medication is vital in HIV management, and Acriptega facilitates this by offering a single tablet solution.
Conclusion
In conclusion, Acriptega is a powerful medication for the management of HIV infection. Its unique combination of Dolutegravir, Lamivudine, and Tenofovir disoproxil fumarate provides effective suppression of the virus while offering the convenience of a single-tablet regimen. While it may have common side effects, their occurrence varies from person to person, and the overall benefits of Acriptega in HIV treatment make it a valuable addition to the arsenal of antiretroviral medications.





 +91-9811604444/ 9811604424/ 9999064250
+91-9811604444/ 9811604424/ 9999064250  8(800)100-47-90
8(800)100-47-90
